Adimmune, your trusted partner to global market
We tailor our services to customers’ needs and offer solutions for various scales, phases, and timelines. We are one of the key suppliers of high-quality vaccines and biologics around the world.
View All
Support Resilient
Supply for Vaccines
We engage with the WHO Pandemic Influenza Preparedness Framework to foster the development and access to vaccines. Recognized by US FDA and the EU, we tackle health challenges by leveraging our high standards of quality.

06 Feb. 2026
Did you know the 1,000 Japanese yen banknote hides a story about the Tetanus Vaccine?
When traveling in Japan, everyone is surely familiar with the 1,000 yen banknote.
The dignified gentleman featured on it is Shibasaburō Kitasato, widely regarded as the “Father of Modern Japanese Medicine”—and a pioneer in therapeutic medicine who helped change the course of human history.
1889: Became the world’s first scientist to successfully culture Clostridium tetani, the bacterium that causes tetanus.
14 Jan. 2026
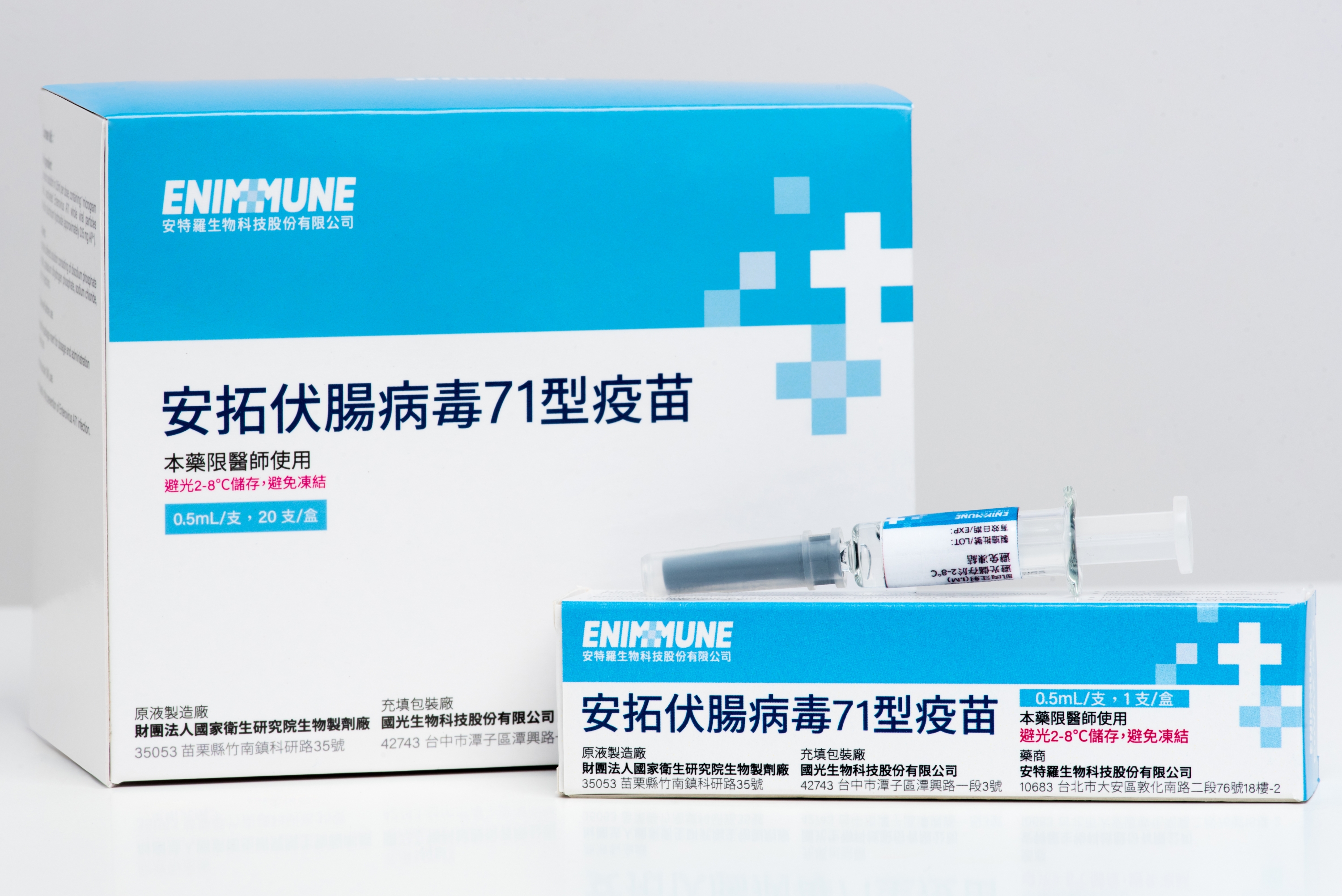
Enimmune’s Enterovirus Vaccine Obtains Vietnam GMP Certification
Enimmune Corp. (TPEx: 6564), an Adimmune Corporation’s subsidiary, announced yesterday through a material information disclosure that it has obtained Vietnam GMP certification for its Enterovirus 71 (EV71) vaccine, EnVAX-A71, which is manufactured at Adimmune’s Tanzi Cell Culture Plant. The certification has been officially published on the website of the Drug Administration of Vietnam (DAV) and Adimmune (TWSE: 4142) is included in the list of GMP-compliant foreign manufacturing facilities.

19 Dec. 2025
Adimmune Celebrates 60th Anniversary with Community-Focused Charity Event
Adimmune Corporation (TWSE: 4142) marked its 60th anniversary yesterday with a charity Christmas fair held in Tanzi District, Taichung, reaffirming the Company’s long-standing commitment to community engagement, public health, and sustainable development.